At present, influenza activity is low in the United States but we expect activity to increase in the coming weeks as the U.S. typically experiences peak influenza activity between December and February. Based on our surveillance data we also expect an increase in norovirus in the very near future. In addition to vaccinations, it is also important to emphasize the early use of antivirals in the treatment of influenza. Antivirals are an important second line of defense, particularly for those at high-risk for complications from influenza. Two neuraminidase inhibitor antiviral medications are recommended for use in the United States—oseltamivir (Tamiflu®) and zanamivir (Relenza®). Current national surveillance data has not revealed significant resistance to oseltamivir and zanamivir to date.
At present, influenza activity is low in the United States but we expect activity to increase in the coming weeks as the U.S. typically experiences peak influenza activity between December and February. Based on our surveillance data we also expect an increase in norovirus in the very near future. In addition to vaccinations, it is also important to emphasize the early use of antivirals in the treatment of influenza. Antivirals are an important second line of defense, particularly for those at high-risk for complications from influenza. Two neuraminidase inhibitor antiviral medications are recommended for use in the United States—oseltamivir (Tamiflu®) and zanamivir (Relenza®). Current national surveillance data has not revealed significant resistance to oseltamivir and zanamivir to date.
Treatment works best when started within the first 48 hours of illness and can shorten the duration of symptoms and reduce  the risk of severe complications and death. Treatment with antiviral medications is recommended for patients with influenza who are hospitalized; have severe, complicated or progressive illness; or are at higher risk for influenza complications. Antiviral treatment may also be considered in other populations, if treatment can be initiated within 48 hours of illness onset. Use of antivirals for the prevention of influenza should be considered for institutional outbreaks (such as in nursing homes or other closed populations) or for those who have contraindications to influenza vaccination. Other preventive health practices that may help decrease the spread of influenza and
the risk of severe complications and death. Treatment with antiviral medications is recommended for patients with influenza who are hospitalized; have severe, complicated or progressive illness; or are at higher risk for influenza complications. Antiviral treatment may also be considered in other populations, if treatment can be initiated within 48 hours of illness onset. Use of antivirals for the prevention of influenza should be considered for institutional outbreaks (such as in nursing homes or other closed populations) or for those who have contraindications to influenza vaccination. Other preventive health practices that may help decrease the spread of influenza and  other common winter illnesses (such as norovirus), include staying home from work and school when ill, staying away from people who are sick, increasing hand washing, and using cough etiquette and respiratory hygiene practices.
other common winter illnesses (such as norovirus), include staying home from work and school when ill, staying away from people who are sick, increasing hand washing, and using cough etiquette and respiratory hygiene practices.
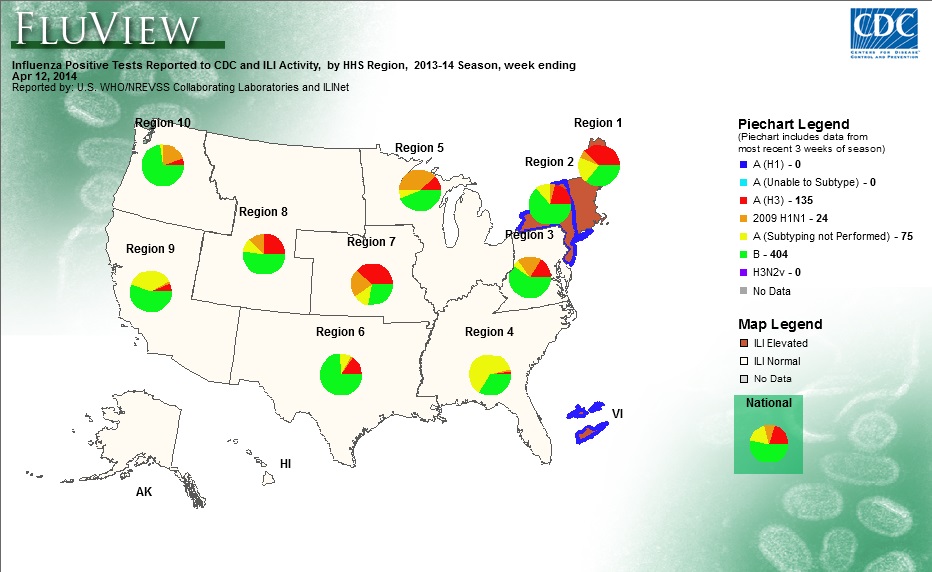
Source: CDC
“The Center has plenty of Quadrivalent Influenza vaccine available.”